Technology Platform: ePurines’ technology platform is based on an understanding of purinergic signaling, mediated by extracellular nucleotides, nucleosides and nucleobases, a rapidly emerging field. This evolving science will assist in the understanding of human (patho)physiology, and provides vast potential for novel drug targets in a variety of disease conditions (Burnstock, 2006; Burnstock, 2011; Burnstock, 2017). ePurines is dedicated to designing, developing and evaluating small molecules that selectively modulate the functions of nucleotide P2 receptors and/or the activity of ecto-nucleotidases, such as CD39, that hydrolyze the ligands of the P2 receptors. Multiple approaches, such as rational drug design and testing, screening of libraries, and the use of Artificial Intelligence and Machine Learning-assisted drug discovery are being used for this.
Innovation: The Scientists at the ePurines have published and/or patented important discoveries related to diseases of the kidney and gastrointestinal system, obesity, organ rejection following transplantation, ischemia-reperfusion injury (Kishore et al, 2015; Kishore et al, 2018; Chia et al 2012; Vaughn et al, 2012; Burnstock et al, 2014; Zeiser et al 2016; Dwyer et al, 2020).
Current Technology: The current technology focuses on targeting P2Y2 purinergic receptor, a G protein-coupled receptor, for the treatment of nephrogenic diabetes insipidus (NDI) as well as obesity. ePurines has an Exclusive License Agreement for the intellectual property from the U.S. Department of Veterans Affairs related to P2Y2 receptor antagonists for the treatment of lithium-induced NDI and related conditions (US Patent # 9,901,624) as well as for the prevention and/or treatment of diet-induced obesity (US Patents #10,024,846 and #10,107,795).
Nephrogenic Diabetes Insipidus: Bipolar disorder affects 5.7 million adults in the United States and it is the sixth leading cause of disability in the world. It is more common among Veterans. Lithium is the only effective drug available to counter the suicidal ideation in patients with bipolar disorder. However, chronic administration of lithium causes nephrogenic diabetes insipidus (NDI), a debilitating kidney condition, for which there is no safe therapy. Development of a potent and safe therapy for NDI will find immediate use in the Veterans Affairs Healthcare System. P2 receptors play significant roles in renal functions, especially in the transport of salt and water (Kishore et al, 2009; Vallon et al, 2020). The Scientists of ePurines discovered that genetic deletion of P2Y2 receptor offers long-term (5 months, equivalent to 20 years in humans) protection against lithium-induced polyuria, natriuresis, kaliuresis, and collecting duct remodeling and proliferation (Zhang et al, 2018). This might be a very significant treatment option for lithium-induced NDI in bipolar Veterans on long-term lithium therapy. In addition, lithium has emerged as a robust neuroprotective agent for the treatment of acute brain injury and chronic neurodegenerative diseases (Rowe and Chuang, 2004; Wada et al, 2005; Quiroz et al 2010; Florenza et al, 2012; Chiu et al, 2013). Evidence suggests that lithium therapy may also be effective in traumatic brain injury (TBI) (Leeds et al, 2014). Currently, the US Department of Veterans affairs is investigating lithium therapy in TBI. Thus, beyond its current use in bipolar disorder, the neuroprotective ability of lithium implies it could be used to treat or prevent brain damage following acute injury, such as ischemic stroke or chronic neurodegenerative diseases, such as Alzheimer’s, Parkinson’s, or Huntington’s disease, as well as TBI. As such the potential use of P2Y2 receptor antagonists in lithium-induced NDI will extend to all these conditions.
Innovation: The Scientists at the ePurines have published and/or patented important discoveries related to diseases of the kidney and gastrointestinal system, obesity, organ rejection following transplantation, ischemia-reperfusion injury (Kishore et al, 2015; Kishore et al, 2018; Chia et al 2012; Vaughn et al, 2012; Burnstock et al, 2014; Zeiser et al 2016; Dwyer et al, 2020).
Current Technology: The current technology focuses on targeting P2Y2 purinergic receptor, a G protein-coupled receptor, for the treatment of nephrogenic diabetes insipidus (NDI) as well as obesity. ePurines has an Exclusive License Agreement for the intellectual property from the U.S. Department of Veterans Affairs related to P2Y2 receptor antagonists for the treatment of lithium-induced NDI and related conditions (US Patent # 9,901,624) as well as for the prevention and/or treatment of diet-induced obesity (US Patents #10,024,846 and #10,107,795).
Nephrogenic Diabetes Insipidus: Bipolar disorder affects 5.7 million adults in the United States and it is the sixth leading cause of disability in the world. It is more common among Veterans. Lithium is the only effective drug available to counter the suicidal ideation in patients with bipolar disorder. However, chronic administration of lithium causes nephrogenic diabetes insipidus (NDI), a debilitating kidney condition, for which there is no safe therapy. Development of a potent and safe therapy for NDI will find immediate use in the Veterans Affairs Healthcare System. P2 receptors play significant roles in renal functions, especially in the transport of salt and water (Kishore et al, 2009; Vallon et al, 2020). The Scientists of ePurines discovered that genetic deletion of P2Y2 receptor offers long-term (5 months, equivalent to 20 years in humans) protection against lithium-induced polyuria, natriuresis, kaliuresis, and collecting duct remodeling and proliferation (Zhang et al, 2018). This might be a very significant treatment option for lithium-induced NDI in bipolar Veterans on long-term lithium therapy. In addition, lithium has emerged as a robust neuroprotective agent for the treatment of acute brain injury and chronic neurodegenerative diseases (Rowe and Chuang, 2004; Wada et al, 2005; Quiroz et al 2010; Florenza et al, 2012; Chiu et al, 2013). Evidence suggests that lithium therapy may also be effective in traumatic brain injury (TBI) (Leeds et al, 2014). Currently, the US Department of Veterans affairs is investigating lithium therapy in TBI. Thus, beyond its current use in bipolar disorder, the neuroprotective ability of lithium implies it could be used to treat or prevent brain damage following acute injury, such as ischemic stroke or chronic neurodegenerative diseases, such as Alzheimer’s, Parkinson’s, or Huntington’s disease, as well as TBI. As such the potential use of P2Y2 receptor antagonists in lithium-induced NDI will extend to all these conditions.

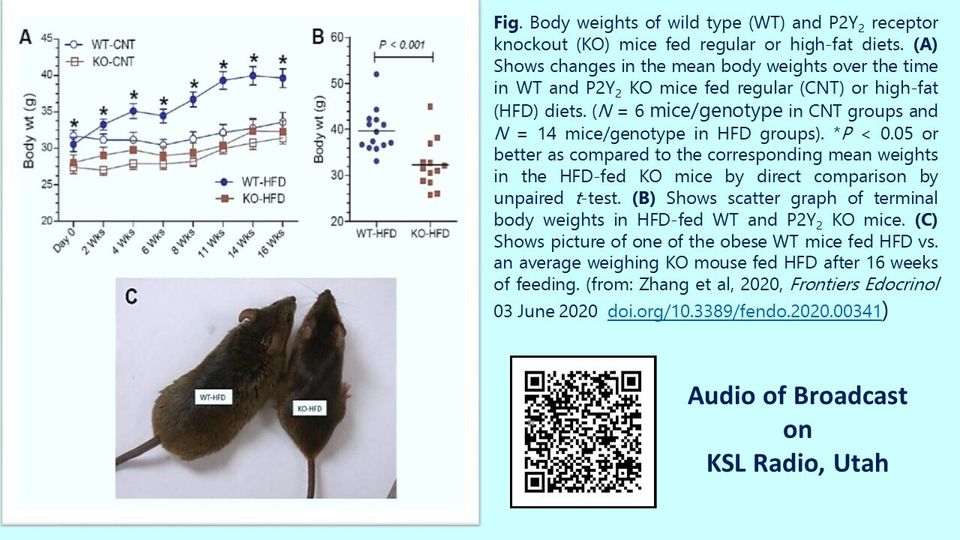
Diet-induced Obesity: According to the World Health Organization 39% or both men and women aged 18 years of above are overweight or obese. In the United States, about 40% of adults are obese, and another 32% overweight. The reported prevalence of obesity and overweight among Veterans is 40 to 73% (Almond et al, 2008). Surprisingly, the combined overweight and obesity in active duty US military personnel rose to more than 60% between 1995 and 2008 (Reyes-Guzman et al, 2015). Overweight and obesity are the prime contributors for the development of type-2 diabetes mellitus (T2DM) and hypertension in addition to several other diseases (Bray, 2004; Pi-Sunyer, 2009), including cancers (Basen-Engquist and Chang, 2011). In June 2013, the American Medical Association officially designated obesity as a “disease”. Diet-induced obesity due to consumption of excessive calories than what one can utilize is the most common cause of obesity worldwide. Thus, there is an unmet need for safe and efficacious anti-obesity drugs that are suitable in all types of subjects. The Global Anti-Obesity Drugs Market Forecast 2020-2030 estimated that the market reached $1.9 billion in 2019 and is expected to grow at a CAGR of 32% in the first half of the forecast period. In 2019, the innovator drugs submarket held 75% of the global anti-obesity drugs market. The on-going COVID-19 pandemic is expected to increase the demand for drugs to promote weight loss, given that obesity is a significant risk factor for case-fatality rates in COVID-19 (Dietz and Santos-Burgoa, 2020). The US Centers Disease Control and Prevention notes that severe obesity (BMI ≥ 40) is associated with higher risk levels for complications due to COVID-19. Obesity could also drive severe complications of COVID-19 disease at younger ages (Kass et al, 2020; Busetto et al, 2020). Similar negative impacts of obesity have been observed in patient outcomes during the H1N1 pandemic of 2009 (Honce and Schultz-Cherry, 2019). Obesity will likely have major impacts on both non-communicable and communicable diseases and may affect global evolutionary trends of influenza other viral diseases. Hence, it is expected that the anti-obesity market will increase substantially over the next several years. The key players in the market are Rhythm Pharmaceuticals, Pfizer, Arena Pharmaceuticals, Vivus, Alizyme, Norgine, Shionogi, GlaxoSmithKline, Orexigen Therapeutics, Merck, Eisai, F. Hoffmann-La Roche, Novo Nordisk, Boehringer Ingelheim, AstraZeneca, Zafgan. The scientists of ePurines have discovered that genetic deletion of P2Y2 receptor confers significant resistance to high-fat diet-induced obesity and insulin resistance in mice. This is achieved by at least five different mechanisms: (Zhang et al, 2020; Zhang et al, Kidney Week 2016 Abstract).
1. decreased adipogenesis and adipose tissue expansion;
2. decreased inflammation in adipose tissue;
3. decreased insulin resistance;
4. increased whole body energy metabolism; and
5. increased sensitivity to browning of fat. Keep the Figure, Legend and the QR Code
Other Uses of P2Y2 Receptor Antagonists: Recent research reports showed that P2Y2 receptor can be targeted to treat a variety of disease conditions that are not related to each other. The same P2Y2 receptor is involved in:
1. Atherosclerosis and restenosis after angioplasty (Seye et al, 2004);
2. Profibrotic responses in cardiac fibroblasts(Braun et al, 2010);
3. Regulation of function of the pericardial fat tissue after cardiac ischemia (Negri et al, 2019);
4. Development of glomerulonephritis, the leading pathology for chronic kidney disease(Rennert et al, 2018);
5. Development of pancreatic ductal adenocarcinoma (Hu et al, 2018).
6. Non-alcoholic Fatty Liver Disease (NAFLD) (Dusabimana et al, 2021)
Thus, targeting P2Y2 receptors holds promise for multiple inflammatory and metabolic conditions
1. decreased adipogenesis and adipose tissue expansion;
2. decreased inflammation in adipose tissue;
3. decreased insulin resistance;
4. increased whole body energy metabolism; and
5. increased sensitivity to browning of fat. Keep the Figure, Legend and the QR Code
Other Uses of P2Y2 Receptor Antagonists: Recent research reports showed that P2Y2 receptor can be targeted to treat a variety of disease conditions that are not related to each other. The same P2Y2 receptor is involved in:
1. Atherosclerosis and restenosis after angioplasty (Seye et al, 2004);
2. Profibrotic responses in cardiac fibroblasts(Braun et al, 2010);
3. Regulation of function of the pericardial fat tissue after cardiac ischemia (Negri et al, 2019);
4. Development of glomerulonephritis, the leading pathology for chronic kidney disease(Rennert et al, 2018);
5. Development of pancreatic ductal adenocarcinoma (Hu et al, 2018).
6. Non-alcoholic Fatty Liver Disease (NAFLD) (Dusabimana et al, 2021)
Thus, targeting P2Y2 receptors holds promise for multiple inflammatory and metabolic conditions

